One Step Dementia Risk Test Kit
Key words:
Qankorey
Classification:
Products
Product Specifications
►First Globally
►Clinically approved
►Early screening: Risk alert of Dementia
►Non-invasive: Fresh midstream urine specimen
►Easy to use: 100 µL (4 drops) volume
►Fast: 10 Mins have result (Invalid after 15 minutes)
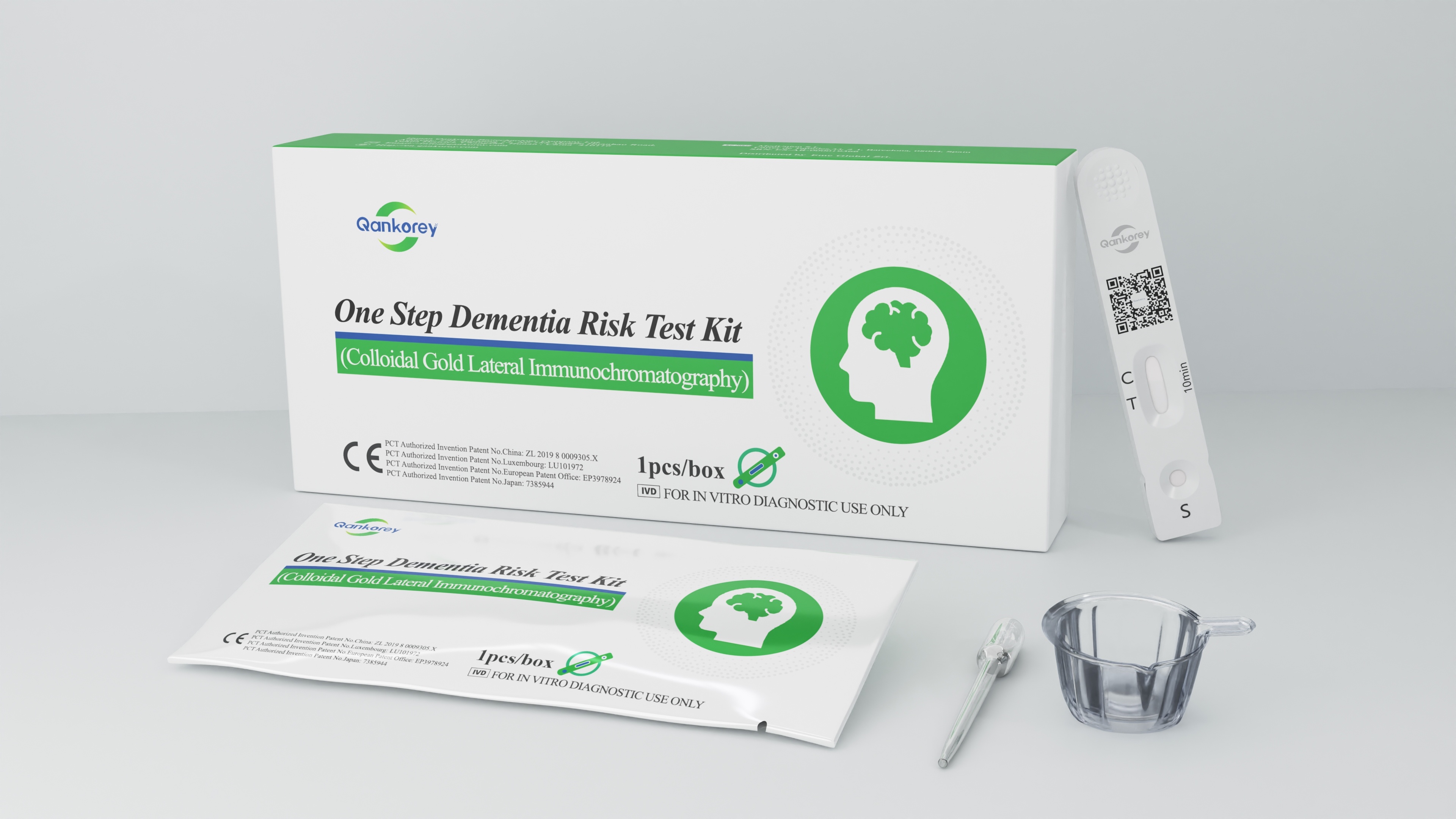
Product Name | One Step Dementia Risk Test Kit (Colloidal Gold Lateral Immunochromatography) |
Certificate | CE, NMPA, MDA |
Packing Specifications | 1pc/box, 25pcs/box, 50pcs/box |
Biomarker to Detect | This is to detect the Aβ concentration in the urine which is the major hallmark of Alzheimer’s Disease. |
Intended Use | This product is an in vitro auxiliary screening device for risk alert of dementia (including but not limited to Alzheimer's disease). It may be used to evaluate brain health status and assist doctors to decide whether further tests are required. |
Key Features | 1. First Globally 2. Clinically approved 3. Early screening: Risk alert of Dementia 4. Non-invasive: Fresh midstream urine specimen 5. Easy to use: 100 µL (4 drops) volume 6. Fast: 10 Mins have result (Invalid after 15 minutes) |
Overall Performance | Sensitivity: 79.14% Specificity: 91.03% Kappa Value: 0.704 |
Applicable People | Suitable for screening high-risk patients aged 45+ |
Product Advantages | Exclusive Core Technology Self-developing of Aβ monoclonal antibody Antibody-polymer sandwich method |
Product Application
It can be applied to the regular physical examination population over 45 years old;
Family history of dementia;
Memory impairment and suspected cognitive impairment;
Cardiovascular and cerebrovascular diseases and cerebrovascular injuries (stroke, cerebral hemorrhage, cerebral infarction, etc.);
Chronic diseases (hypertension, diabetes, hyperlipidemia, etc.), snoring and homocysteine deficiency;
Anxiety, depression, bad habits (smoking and drinking, lack of exercise in the middle of obesity, long-term lack of sleep, etc.);
Recommend Products
A biotechnology company focusing on key technologies for Alzheimer's disease (AD), a global medical challenge.